Which of the Following Best Describes a Double-replacement Reaction
Atoms in one compound switch places with atoms in another compound. Double replacement reaction O acid base reaction.
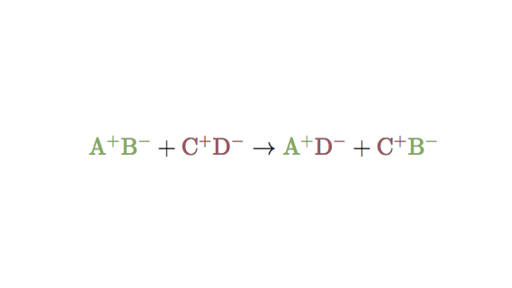
Double Replacement Reactions Double Displacement Article Khan Academy
AgNO3aq NaClaq A AgCl and NaNO3 B AgClO3 and NaNO2 C Ag3N and NaClO3 D AgClO3 and NaNO3 E AgCl and NaNO2.

. An example of a double displacement reaction is the reaction between sodium hydroxide and hydrochloric acid to for sodium chloride as shown. Neutralization precipitation and gas formation are types of double. Which of the following best describes the reaction if any that occurs when aqueous solutions of iron III nitrate and sodium iodide are combined.
2HClaq CaCO3s CO2g CaCl2aq H2Ol. AB A B B. An example would be.
Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. This reaction is considered as a. The the following reaction is.
Ask the player to stop playing the sport Please. What term refers to a chemical reaction that absorbs heat energy. NaOH aq HCl aq.
The correct answer is Option A. PbNO32 2KI -- PbI2 2KNO3 FeS 2HCl -- FeCl2 H2S. Begin chest compressions for CPR D.
Formation of liquid water neutralization rxn Acid Base H₂O ionic compound. The reactants are two compounds and the products are two different compounds. S not obstructed C.
Which of the statements below best describes the following reaction. The symbol h is used to represent heat. The double-replacement reaction below results in the formation of the precipitate lead.
The ions of two compounds exchange places to form two new compounds. Double replacement reactions have two ionic compounds that are exchanging anions or cations. See answer 1 Best Answer.
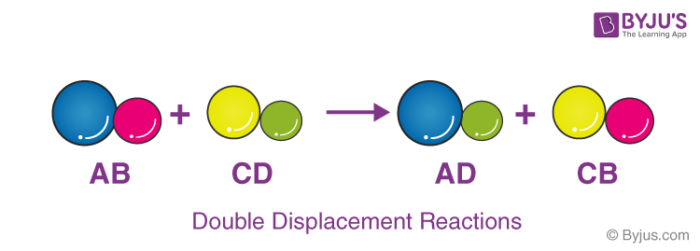
In a double displacement reaction atoms from two different compounds switch places. AX BYAY BX. The ions of two aqueous compounds exchange places to form two new compounds.
Which statement best describes the phase change of a 346 g sample of water vapor to. Identify the precipitate formed when solutions of the following ionic compounds are mixed. Precipitation reactions and neutralization reactions are two common types of double replacement reactions.
HClaq KOHaq KClaq H2Ol. A double replacement reaction is represented by the general equation. The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine.
Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube. The reactant cations are Bismuth Bi and Calcium Ca they switch place in the product. Precipitation reactions produce an insoluble product from two aqueous reactants and you can identify a precipitation reaction using solubility rules.
Draw curved arrows for the following reaction step. This means they recently joined the team. -Formation of liquid water neutralization rxn -Formation of a precipitate.
This reaction is considered as a decomposition reaction because here one substance is breaking into two smaller substances. Fe2O3 6HCl 2FeCl3 3H2O. View the full answer.
Thats why we take the recruitment process seriously to have a. The reaction is made up NaSOU4PoBNF2PoBOU4NaSNF2 The NaS and the PoB switched places. Double displacement reactions can be further classified as.
This group of writers have passed strict English tests plus tests from their fields of specialization. 07202020 Physics High School answered Which of the following best represents a double replacement reaction. SCICHE515 Double-Replacement Reactions - Chemistry.
Write a sentence that completely describes the chemical reaction represented by this balanced equation. See answer 1 Best Answer. Types of DR reactions.
For the given options. DESCRIPTION- the reaction is double displacement reactiondouble replacement reactionmetathesis double. The mass of the reactant is equal to the mass of the product which also means the number of atoms of elements in both the product and reactant are equal.
This reaction is considered as a double displacement reaction because here exchange of ions takes place. Gas Ionic molecular compound 3 more precipitate double-displacement reaction double-replacement reaction. Find an answer to your question Which of the following best represents a double replacement reaction.
Double replacement reaction O acid base. When two elements switch places in a chemical reaction. A reduction and oxidation reaction.
Endothermic double replacement reaction. What are the products from the following double-replacement reaction. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds.
We balance the chemical equation since atoms are not destroyed in the process and the mass is conserved. Which of the following best describes a double-replacement reaction. This is a double replacement reaction that is also a neutralization It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places.
Terms in this set 2 Double Replacement Reactions. Consider the following equation. Mathrm Zn s2 mathrm HCl a q rightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The reaction can be made to occur more slowly by 1 raising the temperature and using a single piece of zinc rather than powdered zinc of the same mass 2.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Which of the following best characterizes this reaction. Exothermic synthesis reaction.
Which of the following reaction is a redox reaction. While the Sodium ion on.

Combustion Reactions Chemical Reactions Chemical Equation Reactions

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
What Is Double Displacement Reaction Chemistry Question

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
Comments
Post a Comment